Author: Dr. Young Il Chang
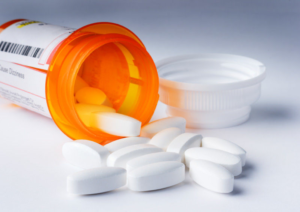
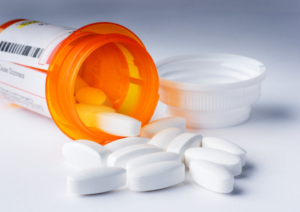
Several companies have initiated recalls of metformin extended-release (ER) tablets due to the possibility of unacceptable levels of N-Nitrosodimethylamine (NDMA), a potential human carcinogen. NDMA, also known as dimethylnitrosamine (DMN), is an organic compound, a volatile yellow oil, which has attracted wide attention due to the high hepatotoxicity and causes liver cancer with chronic exposure in lab animals. In fact, other than the contamination in medications, NDMA is also found in drinking water, cured meat products and can also be formed endogenously following consumption of nitrate-containing food items. For drinking water, it is produced as a byproduct of the water treatment. The acceptable level in California is 10 nanograms per liter in drinking water, but the concern is that it is not readily detectable in this minute concentration and also hard to remove technically.
NDMA was one of several nitrosamine impurities implicated in the recent massive recall of angiotensin II receptor blockers (ARBs), as well as the commonly used H2 antagonists, ranitidine and nizatidine. The recall of variety of valsartan containing products in 2018 was the first instance of a product recall being initiated on the basis of an N-nitroso impurity. The reason for the contamination for valsartan products was considered to be the changes in the synthetic process, which initiated investigation of all -sartans and drugs that may have similar structures or processes. More recently, in April 2020, the FDA required the removal of all ranitidine products from the market after it was discovered that NDMA levels could increase over time under normal storage conditions and that higher levels were associated with the length of time since the product was manufactured. In addition, NDMA was found to increase significantly in ranitidine samples stored at higher temperatures.
According to the FDA, patients who take a drug that contains nitrosamine impurities at or below acceptable daily intake levels (96 nanograms per day) are not expected to be at an increased risk of cancer, even if the drug is ingested daily for 70 years. However, the risk may be increased in individuals who are exposed to it above acceptable levels and for long periods of time. For recent metformin ER contamination, the FDA provides laboratory test results (https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-tests-metformin) and is recommending companies recall lots with levels of NDMA above the acceptable intake limit of 96 nanograms per day.
At this time, the Agency recommends that healthcare professionals continue to prescribe metformin when clinically indicated. Patients should also continue taking metformin ER until advised by their healthcare provider about an alternative treatment option. The immediate-release formulation of metformin has not been found to contain NDMA based on FDA testing. For consumers who are taking the drugs with potential NDMA contaminations, keeping the medication in a storage condition as recommended and consuming it within the expiration date are important to reduce the unnecessary exposure to NDMA.
Imagine your life with the right degree!
REQUEST INFO
SCHOOLS AND PROGRAMS
ADMISSIONS
EVENT LISTS
| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| 29 | 30 | 31 | ||||
Contact AUHS
American University of Health Sciences
1600 E Hill St., Signal Hill CA 90755
T: (562) 988-2278 F: (562) 988-1791
1600 E Hill St., Signal Hill CA 90755
T: (562) 988-2278 F: (562) 988-1791
