Author: Suhui Yang, PhD, Assistant Professor, College of Pharmacy, American University of Health Sciences, Signal Hill, CA 90755
Hepatitis C is an infectious disease affecting primarily the liver and is caused by the Hepatitis C virus (HCV). Chronic infection with HCV is a high-risk factor for developing serious liver diseases, particularly cirrhosis and hepatocarcinoma. HCV infection is a major health burden that affects more than 170 million people around the world, ultimately leading to more than 300,000 deaths per year. Direct-acting antivirals (DAAs), including inhibitors of nonstructural proteins (NS3/4A protease, NS5A, and NS5B polymerase), represent key components of anti-HCV treatment. The combined DAAs therapy has higher efficacy than their monotherapy. However, there are still issues of increased drug resistance, drug-drug interactions, and unwanted side effects. This requires identification of a new class of anti-HCV agents with potential to replace existing regimen.
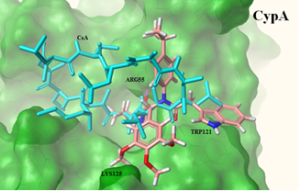
Cyclophilins (Cyps) are a family of highly conserved cellular peptidyl-prolyl cis-trans isomerases (PPIase) and are involved in cellular processes including protein folding and trafficking. Cyclophilin A (CypA) is the most abundant member in the family, and its critical role in supporting HCV-specific RNA replication and viral protein expression has been demonstrated in several reports. To date, most of the existing Cyp inhibitors are derived from two natural products, cyclosporine A (CsA) and sanglifehrin A, thus sharing the same structural backbone. However, the disadvantages of such large CsA-like structures, including high risk of having clinical safety profiles, complex synthetic route, and high cost, have led to identification and development of small-molecule compounds as novel CypA inhibitors for HCV treatment.